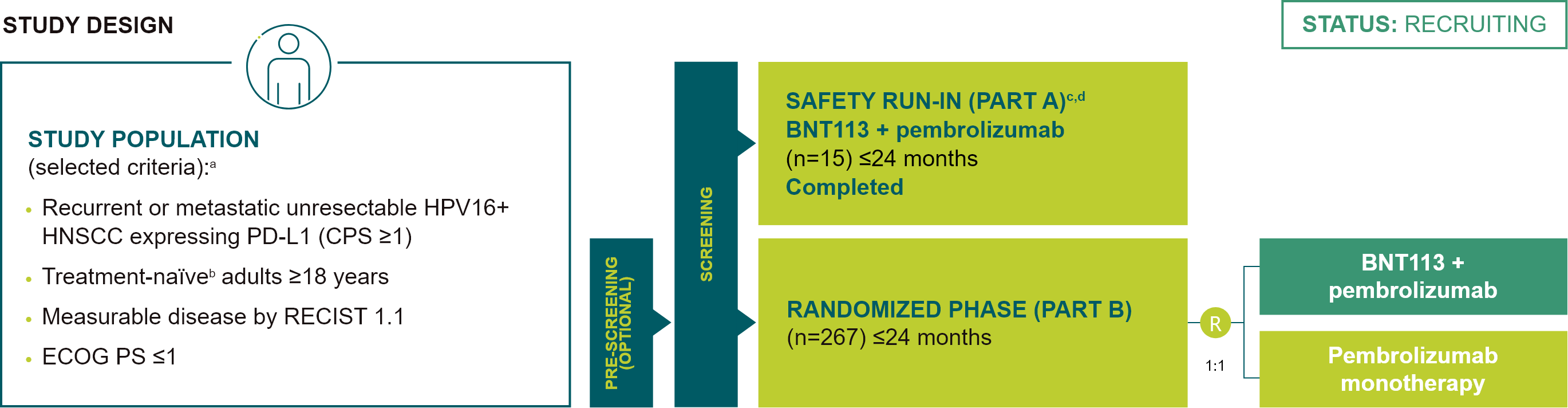
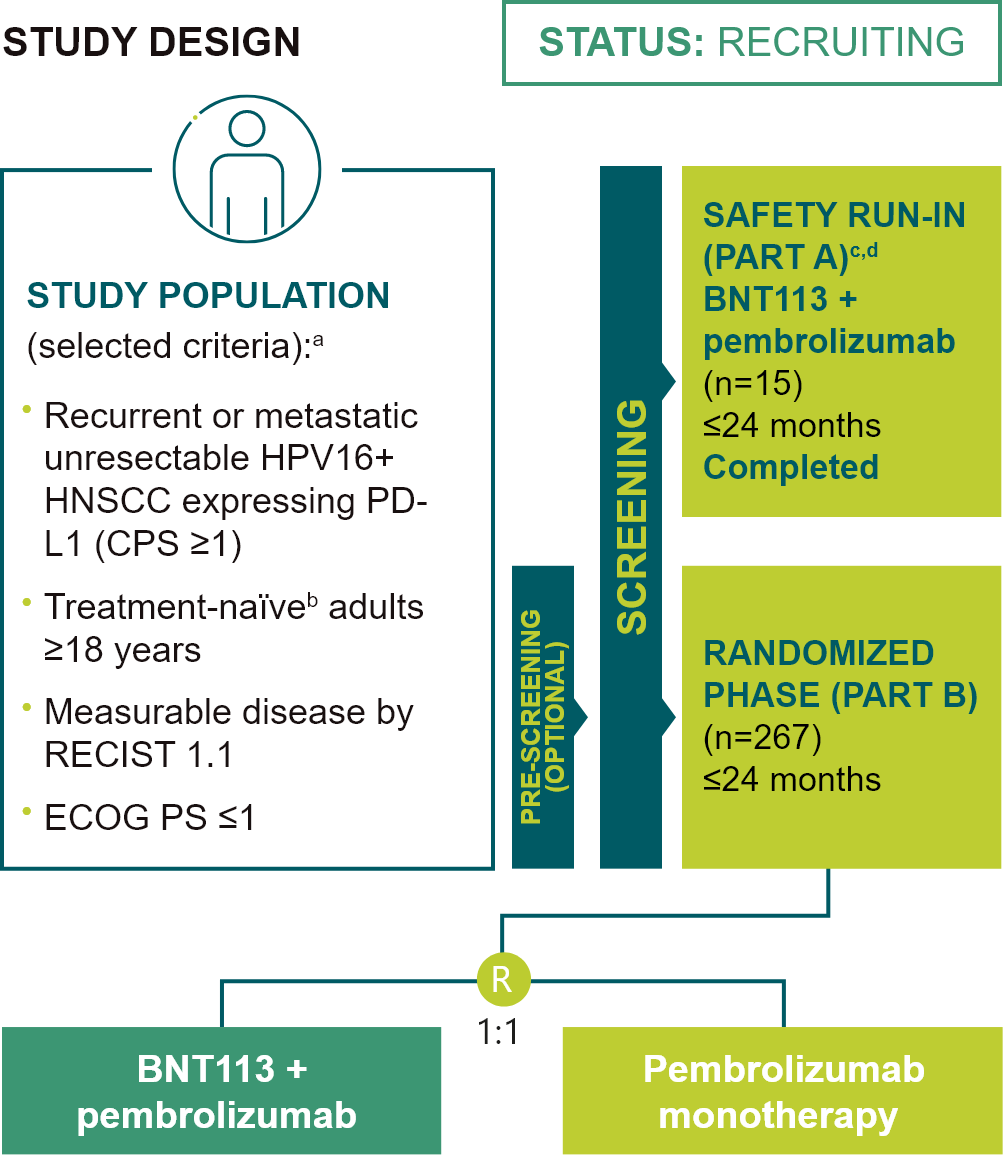
BNT113-01 (AHEAD-MERIT)
A phase II/III randomized trial of BNT113 in combination with pembrolizumab versus pembrolizumab monotherapy as a first line therapy in patients with unresectable recurrent, or metastatic HPV16+, PDL1+ (CPS ≥1) HNSCC (NCT04534205)
Primary Endpoints
- Part A: Occurrence of TEAEs
- Part B: PFS and OS
Secondary Endpoints
- Part A: ORR, DoR, DCR
- Part B: (Key): ORR, PFS by IA, PFS rate at 6 and 12 months by BICR
- Part B: PFS rate at 6 and 12 months by IA, DoR by BICR, TEAEs

For additional details about the BioNTech AHEAD-MERIT trial including study design, study sites, or other information, please visit www.clinicaltrials.gov (Identifier: NCT04534205) or contact the BioNTech team: email, [email protected].
aNot inclusive of all criteria. Refer to https://clinicaltrials.gov/ct2/show/NCT04534205 for more
details; bAs determined by the CE-marked/FDA-approved CDx PD-L1 immunohistochemistry (IHC) 22C3
pharmDx performed according to the manufacturer’s instructions for use; cSystemic therapy which was
completed 180 days prior to randomization, if given as part of multimodal treatment for locally advanced disease
is allowed; dPatients with primary tumor sites of the nasopharynx were excluded; ePatients
included in the Safety Run-In Phase of the trial (Part A) will not be randomized to Part B and will continue
on-trial treatment (BNT113 + pembrolizumab) within Part A until progression or unmanageable toxicity, but for
≤24 months of therapy followed by OS observation; fPreliminary results from the safety run-in set
have been presented at the ESMO Immuno-Oncology Congress 2022, Further details presented at ESMO 2024; Poster
FPN 877P; gThe first BNT113 dose was administered 7 days before the first treatment cycle (pre-cycle
1).
BICR, blinded independent central review; CPS, combined positive score; DCR, disease control rate; DoR, duration
of response; ECOG PS, Eastern Cooperative Oncology Group performance status; HNSCC, head and neck squamous cell
carcinoma; HPV16, human papilloma virus 16; IA, investigator assessment; ORR, overall response rate; OS, overall
survival; PD-L1, programmed cell death ligand 1; PFS, progression-free survival; QxW, every x weeks; RECIST,
Response Evaluation Criteria in Solid Tumors; TEAEs, treatment-emergent adverse events.